Background
Extramedullary plasmacytoma (EMP) is frequently presented in multiple myeloma (MM), especially in relapsed/refractory MM (RRMM), indicating a high-risk state of disease with inferior survival. Even in novel agent era, most anti-myeloma therapeutics demonstrated limited efficacy in EMP. Since there are no randomized controlled studies specifically addressing this complicated situation, standard treatment recommendations still lacked in this setting. Mitoxantrone hydrochloride liposome (Lipo-MIT) has shown higher concentration in tumor tissues and extended half-life, which exhibited clinical benefits in leukemia and solid tumors. Herein we designed a pilot study to explore the efficacy and safety of Lipo-MIT, bortezomib, and dexamethasone (MVD)- based regimen in MM with EMP.
Methods
MM patients with measurable EMP were enrolled in this study. Patients were planned to receive a maximum of 8 cycles of Lipo-MIT, bortezomib, and dexamethasone (MVD)- based regimen. Other combination agents were chosen based on previous treatments and the tolerance of patients, according to the clinician's decision. For patients who were refractory or intolerant to bortezomib, carfilzomib could be an alternative option. The dose of Lipo-MIT was 12mg/m 2 on the first day of every cycle. Both hematological response and EMP response were assessed by the International Myeloma Working Group (IMWG) criteria.
Results
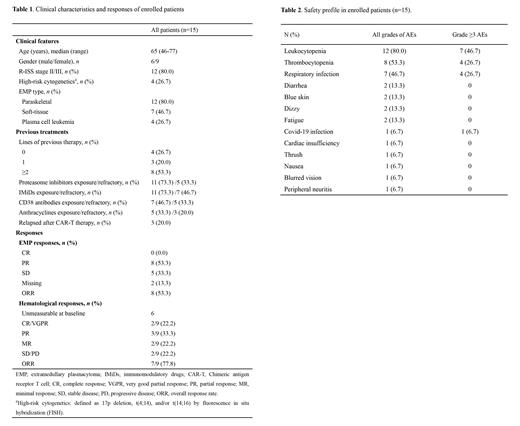
Between Nov 4, 2022 and Jun 25, 2023, fifteen patients were enrolled from Beijing Chaoyang Hospital, with a median age of 65 years (range: 46-77). All patients had at least one kind of EMP, of which 12 (80.0%) patients had paraskeletal plasmacytoma, 7 (46.7%) had soft-tissue plasmacytoma, and 4 (26.7) had plasma cell leukemia. Four (26.7%) patients were newly diagnosed MM (NDMM), three (20.0%) were first relapse, and eight (53.3%) were previously heavily treated (2-12 lines). All of the 11 RRMM patients had been previously treated with proteasome inhibitors and immunomodulatory drugs (IMiDs), seven of them (46.7%) had been treated with CD38 antibodies, five (33.3%) had been treated with anthracyclines, and three (20.0%) had received CAR-T therapy (Table 1).
By the data cut-off date of July 30, 2023, enrolled patients received a median of 2 cycles of Lipo-MIT treatment (range: 1-4). Specific regimens included: Lipo-MIT- bortezomib/carfilzomib- dexamethasone (MVD) triplet regimen (20.0%), MVD plus lenalidomide regimen (M-VRD) (20.0%), MVD plus IMiDs and etoposide regimen (M-VTED) (40.0%), and MVD plus other cytotoxic drugs (cyclophosphamide or cisplatin) (20.0%). The overall response rate (ORR) of EMP was 53.3%. Among the 9 patients who had measurable hematological disease at baseline, the hematological ORR was 77.8% (22.2% ≥VGPR, 33.3% PR, 22.2% MR) (Table 1). The most common adverse effects (AEs) at all grades (incidence >10%) were myelosuppression, including leukocytopenia (80.0%) and thrombocytopenia (53.3%), as well as respiratory infection (46.7%), diarrhea (13.3%), blue skin (13.3%), dizzy (13.3%), and fatigue (13.3%). Common grade ≥3 AEs including leukocytopenia (46.7%), thrombocytopenia (26.7%) and respiratory infection (26.7%) (Table 2). Median follow-up time was 4.5 months. Five patients had died, among which 3 died of disease progression, 1 died of severe respiratory infection, and 1 died of Covid-19 infection. Median PFS and OS have not been reached in this cohort.
Conclusion
This study revealed promising results of early response and efficacy of MVD-based regimen in MM with EMP, which could be further explored in prospective phase 2 studies.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal